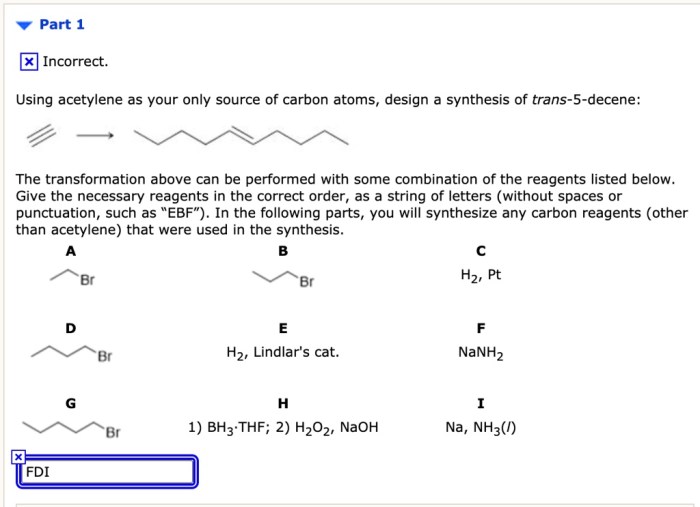
Using acetylene as your only source of carbon atoms opens up a realm of possibilities in chemical reactions, offering distinct advantages and challenges that shape the course of synthetic chemistry.
Acetylene’s unique triple bond imparts remarkable reactivity, enabling a diverse range of transformations that yield valuable compounds and materials. This article delves into the significance, applications, and future prospects of acetylene as a sole carbon source, providing insights into its versatility and potential.
Acetylene as a Sole Carbon Source: Using Acetylene As Your Only Source Of Carbon Atoms
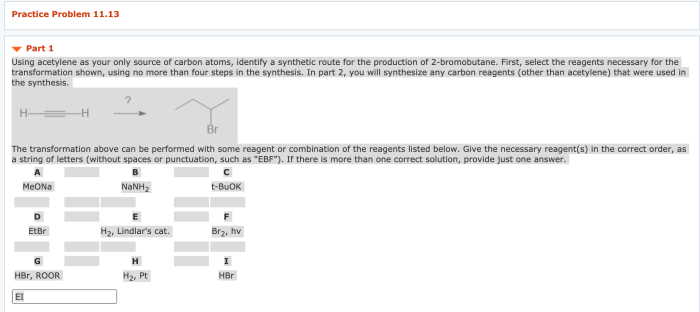
Acetylene, a hydrocarbon with the formula C 2H 2, serves as a significant source of carbon atoms in chemical reactions. Utilizing acetylene exclusively as the carbon source offers unique advantages and disadvantages compared to other carbon sources, influencing its applications in various chemical processes.
Chemical Reactions with Acetylene
Acetylene’s unique triple bond makes it highly reactive, enabling it to participate in diverse chemical reactions. Some notable examples include:
- Hydrohalogenation:Acetylene reacts with hydrogen halides (HX) to form vinyl halides (CH 2=CHX).
- Hydration:In the presence of a catalyst, acetylene reacts with water to form acetaldehyde (CH 3CHO).
- Cycloaddition:Acetylene undergoes cycloaddition reactions with various reagents, such as 1,3-dienes, to form cyclic compounds.
Applications of Acetylene-Based Reactions
Chemical reactions employing acetylene as the sole carbon source have numerous practical applications, including:
- Synthesis of Vinyl Monomers:Acetylene-based reactions are used to produce vinyl monomers, such as vinyl chloride and vinyl acetate, which are essential building blocks for plastics and polymers.
- Production of Acetaldehyde:Acetaldehyde, a key intermediate in the chemical industry, is primarily synthesized through the hydration of acetylene.
- Pharmaceutical Industry:Acetylene-based reactions contribute to the synthesis of various pharmaceutical compounds, such as antibiotics and anti-inflammatory drugs.
Synthetic Strategies with Acetylene
| Reaction Type | Reagents | Conditions | Products |
|---|---|---|---|
| Hydrohalogenation | Acetylene, Hydrogen Halide (HX) | Ambient Temperature, Catalyst | Vinyl Halides (CH2=CHX) |
| Hydration | Acetylene, Water | Catalyst, Pressure | Acetaldehyde (CH3CHO) |
| Cycloaddition | Acetylene, 1,3-Dienes | Heat, Catalyst | Cyclic Compounds |
Challenges and Future Directions, Using acetylene as your only source of carbon atoms
Despite its versatility, using acetylene as the sole carbon source presents certain challenges:
- High Reactivity:Acetylene’s high reactivity can lead to safety concerns and requires careful handling.
- Limited Availability:Acetylene is not naturally abundant and requires industrial production, which can impact its cost and availability.
- Byproducts:Some acetylene-based reactions produce unwanted byproducts, requiring additional steps for their removal.
Future research directions aim to address these challenges through:
- Improved Safety Measures:Developing safer methods for handling and storing acetylene.
- Alternative Sources:Exploring alternative sources of carbon atoms to reduce the reliance on acetylene.
- Selective Catalysis:Designing catalysts that enhance the selectivity of acetylene-based reactions, minimizing byproduct formation.
Helpful Answers
What are the advantages of using acetylene as a sole carbon source?
Acetylene offers several advantages, including its high reactivity due to the triple bond, versatility in forming various functional groups, and ability to access complex molecular architectures.
What are the challenges associated with using acetylene as a sole carbon source?
Acetylene’s high reactivity can also pose challenges, such as the need for controlled reaction conditions to prevent unwanted side reactions and the potential for explosive mixtures when handled improperly.
How is acetylene utilized in practical applications?
Acetylene-based reactions find applications in the synthesis of pharmaceuticals, fragrances, plastics, and other materials. It is also used in welding and cutting processes.