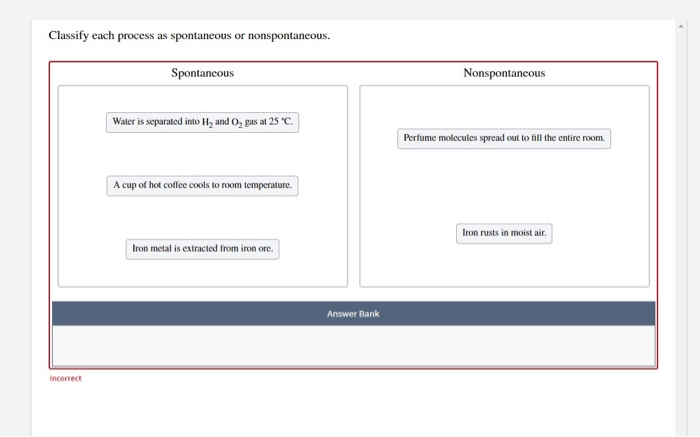
Classify each process as spontaneous or nonspontaneous – Embarking on a journey into the realm of thermodynamics, we delve into the intriguing concept of spontaneous and nonspontaneous processes. These processes govern a myriad of natural phenomena and technological advancements, shaping our understanding of energy transformations and their impact on the world around us.
Spontaneous processes unfold effortlessly, driven by an inherent tendency towards disorder and an increase in entropy. Nonspontaneous processes, on the other hand, require an external energy input to overcome their resistance to change. Understanding the criteria for classifying these processes is crucial for unraveling the intricacies of chemical reactions, biological systems, and a plethora of other real-world applications.
1. Spontaneous Processes
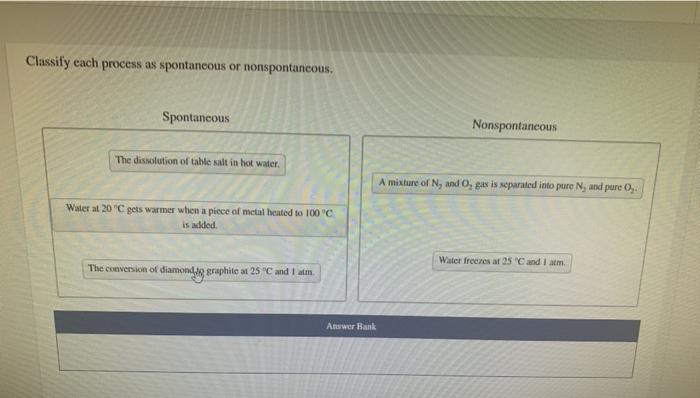
Spontaneous processes are those that occur naturally without any external input of energy. These processes are driven by a decrease in the system’s free energy, which is a measure of the system’s disorder or randomness.
Examples of spontaneous processes include:
- Diffusion of gases
- Dissolution of sugar in water
- Rusting of iron
The factors that contribute to spontaneity include:
- Entropy: The measure of disorder or randomness in a system. Spontaneous processes tend to increase entropy.
- Enthalpy: The measure of the heat content of a system. Spontaneous processes tend to decrease enthalpy.
- Free energy: The measure of the system’s tendency to undergo change. Spontaneous processes have a negative free energy change.
2. Nonspontaneous Processes
Nonspontaneous processes are those that do not occur naturally and require an external input of energy to proceed. These processes are driven by an increase in the system’s free energy.
Examples of nonspontaneous processes include:
- Freezing of water
- Melting of ice at room temperature
- Electrolysis of water
The role of external energy in nonspontaneous processes is to provide the energy needed to overcome the increase in free energy. This energy can be provided in the form of heat, light, or electricity.
3. Criteria for Classifying Processes
| Characteristic | Spontaneous Processes | Nonspontaneous Processes |
|---|---|---|
| Entropy change | Increase | Decrease |
| Enthalpy change | Decrease | Increase |
| Free energy change | Negative | Positive |
The flowchart below illustrates the decision-making process for classifying processes:
- Start with the initial state of the system.
- Determine whether the process is spontaneous or nonspontaneous based on the criteria above.
- If the process is spontaneous, it will proceed naturally.
- If the process is nonspontaneous, it will require an external input of energy to proceed.
Understanding the criteria for classification is important because it allows us to predict whether a process will occur naturally or not. This knowledge can be used to design and control chemical reactions, as well as to understand the behavior of natural systems.
4. Real-World Applications
The classification of processes is applied in various fields, including:
- Chemistry: To design and control chemical reactions.
- Biology: To understand the behavior of biological systems.
- Engineering: To design and control industrial processes.
- Environmental science: To understand and mitigate the impact of human activities on the environment.
For example, in chemistry, the classification of processes is used to design and control chemical reactions. By understanding the spontaneity of a reaction, chemists can predict whether it will occur naturally or not. This knowledge can be used to design reactions that are more efficient and environmentally friendly.
In biology, the classification of processes is used to understand the behavior of biological systems. For example, the spontaneous diffusion of gases is essential for the transport of oxygen and carbon dioxide in the body. Understanding the spontaneity of these processes can help us to understand how the body functions and to develop new treatments for diseases.
5. Limitations and Exceptions: Classify Each Process As Spontaneous Or Nonspontaneous

The criteria for classifying processes are general guidelines and there are some exceptions to the rules. For example, some processes that appear to be spontaneous actually require a small input of energy to initiate them. These processes are called “activated processes.”
Another limitation of the criteria is that they do not take into account the effects of catalysts. Catalysts are substances that can speed up the rate of a reaction without being consumed. The presence of a catalyst can make a nonspontaneous process spontaneous.
It is important to be aware of the limitations of the criteria for classifying processes and to use them with caution. In some cases, it may be necessary to conduct experiments to determine whether a process is spontaneous or nonspontaneous.
FAQ Summary
What are the key factors that determine spontaneity?
The change in entropy, enthalpy, and Gibbs free energy are the primary factors that govern spontaneity.
How can we predict the spontaneity of a reaction?
By calculating the change in Gibbs free energy, we can determine whether a reaction is spontaneous or nonspontaneous under specific conditions.
What is the role of catalysts in spontaneous and nonspontaneous processes?
Catalysts can accelerate the rate of both spontaneous and nonspontaneous processes by providing an alternative pathway with a lower activation energy.