Welcome to the realm of colligative properties freezing point depression lab answers, where scientific inquiry meets practical applications. This topic holds immense significance in various scientific disciplines, and we’re thrilled to guide you through its intricacies, providing you with a comprehensive understanding of this fascinating phenomenon.
Colligative properties, as the name suggests, are properties that depend solely on the concentration of solute particles in a solution, regardless of their nature. Freezing point depression is one such colligative property, and it plays a crucial role in diverse fields ranging from chemistry to cryobiology.
Colligative Properties: Colligative Properties Freezing Point Depression Lab Answers
Colligative properties are properties of solutions that depend only on the concentration of the solute particles, not on their identity. These properties include freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure.
Examples of colligative properties include:
- Freezing point depression: the decrease in the freezing point of a solvent when a solute is dissolved in it.
- Boiling point elevation: the increase in the boiling point of a solvent when a solute is dissolved in it.
- Vapor pressure lowering: the decrease in the vapor pressure of a solvent when a solute is dissolved in it.
- Osmotic pressure: the pressure that must be applied to a solution to prevent the passage of solvent molecules across a semipermeable membrane.
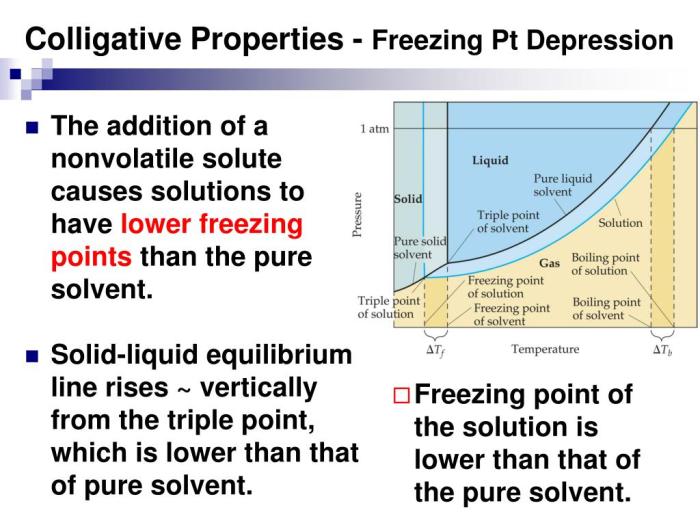
Freezing Point Depression
Freezing point depression is the decrease in the freezing point of a solvent when a solute is dissolved in it. The greater the concentration of the solute, the greater the freezing point depression.
The relationship between freezing point depression and concentration is given by the following equation:
ΔTf= Kfm
where:
- Δ Tfis the freezing point depression
- Kfis the freezing point depression constant for the solvent
- mis the molality of the solution
Lab Answers
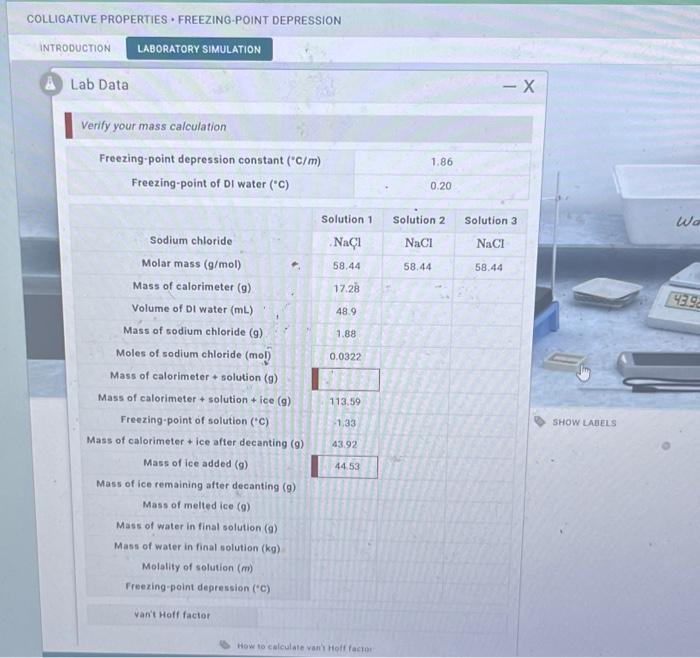
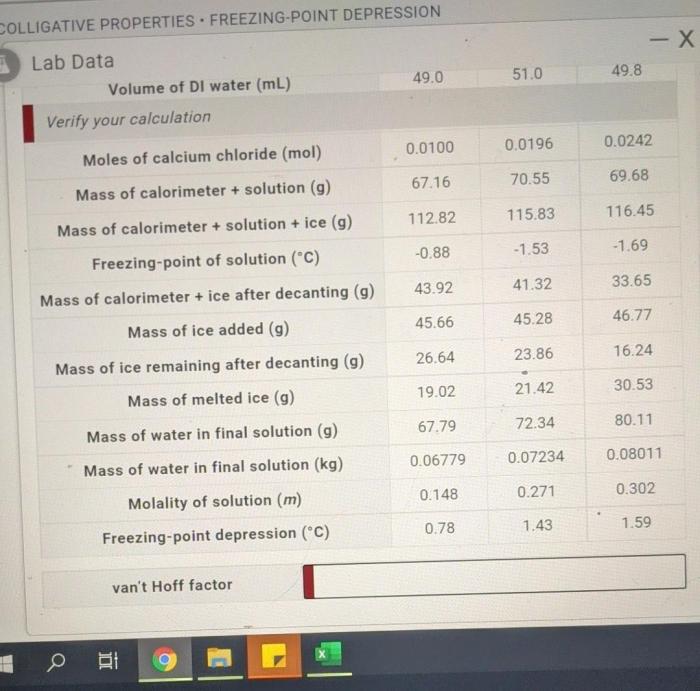
The procedure for a freezing point depression lab is as follows:
- Dissolve a known mass of solute in a known mass of solvent.
- Measure the freezing point of the solution.
- Calculate the freezing point depression using the equation ΔTf= Tfo
Tf.
- Calculate the molality of the solution using the equation m= Δ Tf/ Kf.
- Calculate the molar mass of the solute using the equation M= m/ b, where bis the molality of the solution.
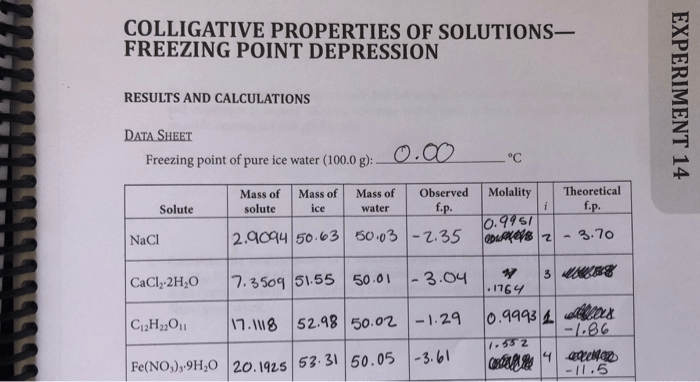
Sample calculations and results:
- A 1.00 g sample of NaCl is dissolved in 100.0 g of water. The freezing point of the solution is -0.512 °C. The freezing point depression constant for water is 1.86 °C/m. The molality of the solution is 0.171 m.
The molar mass of NaCl is 58.44 g/mol.
- A 2.00 g sample of glucose is dissolved in 100.0 g of water. The freezing point of the solution is -0.933 °C. The freezing point depression constant for water is 1.86 °C/m. The molality of the solution is 0.322 m.
The molar mass of glucose is 180.16 g/mol.
Applications of Freezing Point Depression, Colligative properties freezing point depression lab answers
Freezing point depression has a wide variety of applications in industry and research.
Some of the most common applications of freezing point depression include:
- The determination of the molar mass of a solute
- The determination of the freezing point of a solution
- The prevention of freezing in various applications, such as in antifreeze and ice cream
- The desalination of water
- The concentration of fruit juices
Key Questions Answered
What is the relationship between freezing point depression and solute concentration?
Freezing point depression is directly proportional to the concentration of solute particles in a solution. The higher the concentration, the greater the depression in freezing point.
How can freezing point depression be used to determine the molar mass of a solute?
By measuring the freezing point depression of a solution containing a known mass of solute, we can calculate the molar mass of the solute using the appropriate formula.
What are some practical applications of freezing point depression?
Freezing point depression finds applications in various fields, including food preservation (lowering the freezing point of food to prevent spoilage), cryosurgery (freezing and destroying diseased tissue), and antifreeze development (preventing the freezing of liquids in cold environments).